Food labeling transcends mere legal formality; it stands as a fundamental pillar of consumer trust, public health, and market access in the global economy. For manufacturers, navigating the increasingly intricate web of international regulations presents a significant challenge. The stakes are profoundly high, as non-compliance can lead to severe consequences, including costly product recalls, substantial financial penalties, irreversible brand damage, and critical consumer health risks. This comprehensive guide aims to demystify global food labeling requirements, elucidate the inherent risks, and demonstrate how leveraging advanced technology, particularly sophisticated labeling machines and integrated packaging lines, can ensure compliance and drive sustained business success.
Achieving global market presence necessitates a deep understanding of diverse regulatory frameworks. While a core intent of transparency is universal, the precise execution of compliance is highly localized, creating a “global-local” paradox for manufacturers. This demands agile systems capable of adapting to precise regional specifications, rendering a “one-size-fits-all” labeling approach impractical for international distribution. Regulatory bodies are not static entities; they actively respond to evolving scientific understanding, consumer awareness, and societal health priorities, meaning manufacturers must anticipate that consumer trends will eventually translate into new regulatory requirements.

The U.S. FDA: Decoding Clarity and Consumer Information
In the United States, the Food and Drug Administration (FDA) is tasked with ensuring that foods sold are safe, wholesome, and properly labeled under the Federal Food, Drug, and Cosmetic (FD&C) Act and the Fair Packaging and Labeling Act. Adherence to these regulations is a legal imperative and a vital step in safeguarding consumer trust and safety.
Mandatory elements for food labels in the U.S. include the common name or identity of the food, which must be prominently displayed on the Principal Display Panel (PDP) and clearly describe the product’s nature, such as “Chocolate” rather than just a brand name. This statement of identity should be in bold type, generally at least half the size of the largest print on the label. The net quantity of contents, indicating the product amount by weight, volume, or count, must also appear on the PDP, prefixed by “Net Weight” and occupying the bottom 30% of the panel.
A comprehensive ingredient list is mandatory, with ingredients listed by weight in descending order. This can be on the PDP or the adjacent information panel, and sub-ingredients may be nested. Crucially, artificial colors or flavors must be clearly disclosed. The name and location of the manufacturer, packer, or distributor are also required, ideally on the PDP or information panel. If the entity listed is not the actual manufacturer, a qualifying phrase like “Manufactured for [Your Company Name]” is necessary.
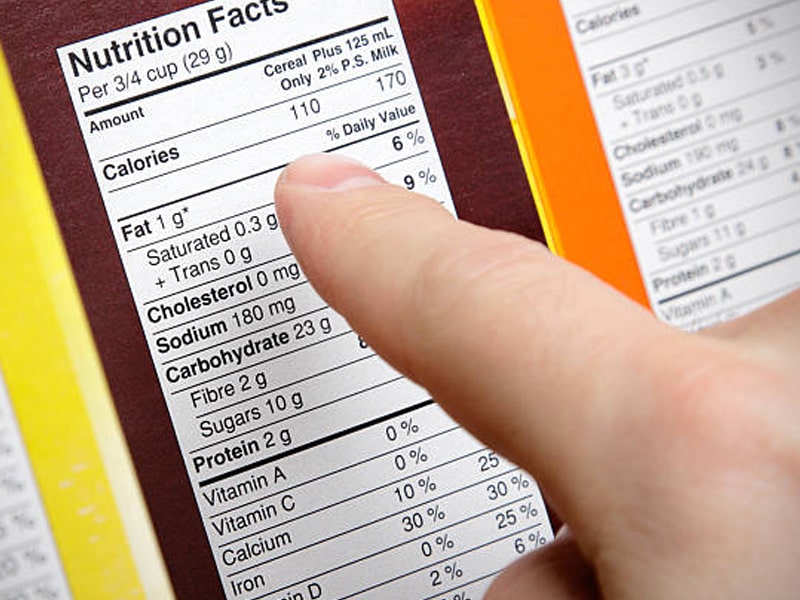
Nutrition information, commonly known as the Nutrition Facts Label, is typically found on the rear panel and details nutrients, vitamins, minerals, and macronutrients. This label is generally required for products intended for retail sale, especially if any health or nutrient claims are made. Recent FDA updates to the Nutrition Facts label emphasize calorie counts (now larger and bolded), include “Added Sugars” in grams and as a percentage of daily value, and feature revised serving sizes to reflect current dietary guidelines.
Legibility and placement are paramount. All information must be prominent, conspicuous, and easy to read, with sufficient contrast against the background. The FDA specifies minimum font sizes: at least 1/16 inch for the lowercase ‘o’ on the information panel. For the Nutrition Facts label, specific font sizes apply, such as a minimum of 6 points for footnotes, 8 points for most text, 16 points for “Calories” (10 points for small packages), and 22 points for the numerical calorie value. Sans-serif fonts like Arial or Helvetica are preferred to ensure clarity.
Certain claims are prohibited or highly scrutinized. Unverified health claims, such as “heart-healthy” without FDA authorization, are not permitted. Nutrient content claims, like “low in fat” or “zero sugar,” must meet specific FDA criteria and be substantiated by the Nutrition Facts Panel. The term “natural” is particularly ambiguous and frequently litigated, as it lacks a federal definition under FDA regulations, often leading to consumer confusion and potential legal challenges. It is important to note that the FDA does not pre-approve labels for food products.
The European Union (EU): Harmonization with Specificity
The European Union’s food labeling regulations are primarily based on the Food Information Regulation (EU) No 1169/2011 (FIC), which is directly applicable in all member states but can be complemented by national laws.
Mandatory elements under EU regulations include the name of the food, which must precisely clarify the product’s nature, using legally prescribed designations where they exist. A list of all ingredients is required, presented in descending order of weight at the time of production. Food additives and flavorings must be included, typically with their category name (e.g., “colour”) followed by their specific name or E-number (e.g., “curcumin” or “E 100”).
Allergen labeling is a critical component, requiring the 14 most important substances causing allergies or intolerances (e.g., nuts, soy) to be emphasized within the ingredient list through font type, style (e.g., bold), or background color. Precautionary allergen labeling (PAL) is also a consideration, although not formally regulated, general food safety principles apply. The net quantity of the product, whether by number of items, weight (g/kg), or volume (ml/l), must be indicated.
The best-before date signifies how long a food retains its specific characteristics if stored correctly, while a use-by date is required for highly perishable foodstuffs, indicating when they become unsafe for consumption. The name and address of the company responsible for the product must also be present. Origin marking is necessary when consumers might be misled about the actual origin, especially if the primary ingredient’s origin differs from the product’s.
A mandatory nutritional declaration, often referred to as the “Big 7,” has been required since December 2016. This declaration typically appears as a table and includes energy value (kJ/kcal), fat, saturates, carbohydrates, sugars, protein, and salt, with values expressed per 100g or 100ml. Vitamins and minerals are only included if present in significant amounts and expressed as a percentage of the daily reference intake. Other specific requirements include instructions for use if a foodstuff would be difficult to use appropriately, alcoholic strength for beverages exceeding 1.2% volume, specific labeling for “formed meat” or “formed fish,” and warnings for foods containing high caffeine. All engineered nanomaterials used as ingredients must be indicated with the word ‘nano’ in brackets.
Legibility standards dictate that mandatory information must be easily visible, clearly legible, and indelible, with a minimum font size where the height of the lower-case ‘x’ is at least 1.2 mm (0.9 mm for small packaging under 80 cm²). Recent updates include the compulsory display of an ingredient list and nutrition declaration for wine since December 2023. Additionally, Germany, as a member state, now requires the country of rearing and slaughter for certain fresh, chilled, or frozen meats as of February 2024.
China’s Evolving Landscape: Stricter Standards for Transparency
China’s food labeling landscape is undergoing significant transformation with the finalization of new Food Labeling and Marking Supervision and Management Measures on March 14, 2025, set to take effect on March 16, 2027. These measures will replace older regulations and are complemented by new national standards (GB 7718-2025 and GB 28050-2025).
Mandatory labeling items must be in standard Chinese characters, although Traditional Chinese characters, Pinyin, and foreign languages are permitted, provided their font size does not exceed that of the standard Chinese characters. Food names must accurately reflect the true nature of the foods and avoid misleading consumers. Manufacturer information, including name and address, must match the food production license, and contact details must be valid. For contract-manufactured foods, details of both the entrusting and entrusted parties are required.
The ingredient list must use “Ingredients” or “Ingredient List” as guiding language, and repackaged foods must be clearly marked as “repackaged”. Net content for quantitative prepackaged foods must comply with specific measures, with units (volume, mass, length) prescribed based on the food’s state. For foods with solid and liquid phases where the solid is the main ingredient, drained matter content must also be labeled.
Production and shelf-life expiration dates must be marked in a dedicated, high-contrast area (e.g., black text on a white background) and not be prone to falling off. Manufacturers can omit the production date for products with a shelf life exceeding six months. China’s nutrition labeling standard has expanded from a “1+4” system (energy, protein, fat, carbohydrate, sodium) to a “1+6” format, now mandating the disclosure of saturated fats and total sugars. Warnings regarding excessive intake of salt, oil, and sugar, particularly aimed at children and adolescents, must also be included.
A significant move to enhance food safety for allergy sufferers is the mandate for clear highlighting (bolding, underlining, or a clear statement below the list) of eight major allergens: gluten-containing cereals, crustaceans, fish, eggs, peanuts, soybeans, milk, and tree nuts. However, authorities caution against “allergen-free” labels due to the scientific infeasibility of guaranteeing complete absence and the variability of individual allergic reactions.
Legibility and placement requirements specify that texts, symbols, numbers, and graphics must clearly contrast with the background color. Font height for mandatory labeling items must be no smaller than 1.8 mm, with a height-to-width ratio not exceeding 3. Larger packaging has stricter requirements: 2.0 mm for surfaces over 150 cm² and 2.5 mm for over 400 cm². Production and shelf-life dates have an even larger minimum font height of 3.0 mm (2.0 mm for packages smaller than 35 cm²).
Prohibited content includes claims relating to disease prevention or treatment, false, deceptive, misleading, or exaggerated descriptions, and terms such as “no additives” or “zero additives,” which Beijing contends may mislead consumers. China has also introduced a groundbreaking digital labeling system, allowing consumers to scan QR codes on packaging for expanded product information via mobile devices, addressing concerns regarding small font sizes and enhancing accessibility, particularly for older consumers.
Comparative Overview of Key Food Labeling Requirements (US, EU, China)
The following table provides a concise comparison of key food labeling requirements across the United States, European Union, and China. This overview is invaluable for manufacturers operating or planning to operate in multiple global markets, offering a quick reference to complex regulatory landscapes and highlighting both commonalities and critical differences. This helps busy professionals quickly grasp the nuances for initial compliance checks and strategic planning for global product launches.
| Labeling Element | US FDA (United States) | EU (European Union) | China (SAMR) |
| Common Name/Identity | Prominently displayed on PDP, bold, ≥1/2 size of largest print. Must clarify product nature. | Must clarify precise nature, legally prescribed designation. | Must reflect true nature, not mislead. |
| Net Quantity | On PDP, “Net Weight” prefix, bottom 30% of PDP. Weight, volume, or count. | Number of items, weight (g/kg), or volume (ml/l). | Specific units (volume/mass/length) based on food state. Drained matter for solid/liquid phase foods. |
| Ingredient List | Descending order by weight. PDP or info panel. Sub-ingredients nested. Artificial colors/flavors disclosed. | Descending order by weight. Additives/flavorings with category/E-number. | “Ingredients” or “Ingredient List” as guiding language. “Repackaged” label if applicable. |
| Nutrition Information | Nutrition Facts Label. Emphasizes calories, “Added Sugars.” Required for most retail products, especially if claims made. | “Big 7” (energy, fat, saturates, carbs, sugars, protein, salt) per 100g/100ml. Mandatory since Dec 2016. | Expanded “1+6” (energy, protein, fat, carb, sodium, saturated fats, total sugars). Warnings for excessive salt/oil/sugar. |
| Allergen Labeling | Part of ingredient list. No specific emphasis mandate, but clear disclosure. | 14 major allergens emphasized (bold, font, color) in ingredient list. PAL considered. | 8 major allergens highlighted (bold, underline, or statement). Caution against “allergen-free.” |
| Font Size Minimums | Info panel: 1/16 inch (lowercase ‘o’). Nutrition Facts: 6pt (footnotes), 8pt (most), 16pt (Calories), 22pt (Calories numerical). | Lower-case ‘x’ at least 1.2 mm (0.9 mm for <80 cm²). | Mandatory items: 1.8 mm (height-to-width ratio ≤3). Larger for bigger packaging. Dates: 3.0 mm (2.0 mm for <35 cm²). |
| Manufacturer Info | Name & location of manufacturer/packer/distributor. “Manufactured for” if applicable. | Name & address of responsible company. | Name & address match production license. Contact info valid. Both parties for contract manufacturing. 10 |
| Dates | No specific format mentioned in snippets. | Best-before date / Use-by date. | Production date & shelf-life expiration date. High-contrast, dedicated area. Can omit production date for shelf life >6 months. |
| Prohibited Claims | Unverified health claims. Nutrient claims must meet FDA criteria. “Natural” is ambiguous. | No specific list in snippets, but general food information must not mislead. | Claims for disease prevention/treatment. False, deceptive, misleading, exaggerated. “No additives” / “Zero additives” prohibited. |
| Special Notes/Updates | FDA does not pre-approve labels. | Wine now requires ingredient list/nutrition declaration (Dec 2023). Germany: meat origin (Feb 2024). Nanomaterials ‘nano’ in brackets. | New Measures effective March 2027. Digital labeling (QR codes) for expanded info. |
The Perilous Path: Risks and Repercussions of Non-Compliance
Failing to meet food labeling requirements carries severe consequences that extend far beyond simple fines, posing fundamental threats to a company’s viability. The ripple effect of mislabeling can cascade from immediate regulatory penalties to existential threats, underscoring the need for a preventative, rather than reactive, approach to compliance.
Safeguarding Public Health: The Dire Consequences of Mislabeled Allergens and Nutrients
One of the most critical risks associated with mislabeling is the potential exposure of consumers to common allergens. Mislabeled allergens can lead to severe allergic reactions, including life-threatening anaphylaxis. There are over 160 known food allergens, with specific ones requiring explicit declaration on labels. For instance, regulations in Canada mandate the declaration of ten key allergens and gluten sources if present in a product or facility.
Beyond allergens, incorrect nutritional information can also lead to significant health hazards. If a product is erroneously labeled as sugar-free but contains sugar, individuals with diabetes consuming it may face severe health complications. Similarly, mislabeling a food item as gluten-free when it contains gluten can cause adverse reactions in individuals with gluten sensitivity. Such mislabeling also raises profound ethical concerns regarding consumer trust and transparency, particularly when products are deceptively marketed, for example, as “organic” without proper certification.
Legal and Financial Ramifications: Fines, Recalls, and Litigation
Non-compliance can trigger a host of legal and financial repercussions. Regulatory bodies like the FDA or FTC can impose civil penalties, including fines, product recalls, and mandatory corrective measures. The FDA, for example, has the authority to detain or reject shipments at the border if labeling is inconsistent or misleading. In cases of deliberate mislabeling, especially those involving false or deceptive claims, companies or even individuals responsible may face criminal charges.
Furthermore, companies are vulnerable to consumer litigation. Class-action lawsuits, particularly prevalent in states like California, New York, and New Jersey, can arise if unverified claims (e.g., advertising a product as “organic,” “kosher,” or “halal” without proper certification) mislead consumers into paying premium prices. These legal actions can result in substantial financial damages for the company. A single contamination incident or mislabeling error can also initiate costly product recalls, leading to millions of dollars in lost revenue, legal fees, and operational disruption. Robust traceability systems, however, can help mitigate the financial impact by enabling targeted recalls of only affected batches.
Erosion of Trust: Protecting Your Brand Reputation
The consequences of mislabeling extend deeply into a company’s brand reputation. Dishonest labeling fundamentally undermines consumer trust in the brand and, by extension, in the broader food industry. Once this trust is eroded, it becomes exceedingly difficult to regain. Consumers who lose faith in a brand due to mislabeling are likely to switch to competitors, leading to enduring financial implications and a tarnished brand image.
Misrepresenting product quality, such as substituting listed ingredients with cheaper alternatives, directly damages the brand’s perceived value and integrity. In the age of social media, public perception can shift overnight, making rapid and precise handling of any labeling issues imperative. High-profile incidents like the European Horsemeat Scandal in 2013 or the Spanish olive oil fraud in 2016 serve as stark reminders of the severe consequences of dishonest labeling, which included massive recalls and significant financial losses. These cases illustrate that the damage to reputation can be existential, leading to long-term loss of business.
A particular challenge arises from the ambiguity surrounding terms like “natural.” This term is widely used yet remains one of the most ambiguous and litigated claims, lacking a federal definition in the U.S.. This ambiguity is not merely a regulatory oversight; it is a direct pathway to consumer deception and subsequent litigation. The absence of a clear definition means even well-intentioned manufacturers can inadvertently fall short of consumer expectations or legal interpretations. China’s proactive prohibition of “no additives” or “zero additives” claims demonstrates a regulatory response to this ambiguity, aiming to prevent consumer confusion. This highlights that manufacturers must move beyond simply avoiding explicitly false claims; they must adopt a principle of radical transparency and scientific substantiation for all claims, especially those that resonate with current consumer trends. Relying on ambiguous terms is a high-stakes gamble that can undermine trust and invite legal challenges, even if no explicit regulation is technically violated.
Common Labeling Non-Compliance Issues and Their Impacts
Understanding common pitfalls and their direct consequences is crucial for manufacturers to identify vulnerabilities and prioritize improvements in their labeling processes. This table reinforces the critical need for investing in robust compliance solutions.
| Non-Compliance Issue | Specific Error/Issue | Immediate Consequence | Broader Impact |
| Misleading Health/Nutrient Claims | “Heart-healthy” without FDA approval; “low fat” without meeting criteria; “with vitamin D” but only 5% DV. | Product detainment/rejection at border; Fines; Regulatory intervention. | Consumer litigation (class-action lawsuits); Erosion of consumer trust; Brand reputation damage. |
| Inaccurate Ingredient Lists | Missing ingredients; Incorrect order by weight; Undisclosed artificial colors/flavors; Ingredient substitution (e.g., cheaper oils). | Product recall; Fines. | Allergic reactions; Health hazards (e.g., for diabetics); Loss of consumer trust; Legal liabilities. |
| Illegible/Poorly Placed Text | Font too small; Insufficient contrast; Information obscured by artwork; Key details not on PDP. | Misbranding violation; Regulatory action. | Consumer confusion; Reduced accessibility; Perceived lack of transparency; Brand image degradation. |
| Mislabeled Allergens | Failure to declare major allergens (e.g., nuts, soy); Incorrect emphasis in ingredient list. | Product recall; Fines; Regulatory intervention. | Severe allergic reactions (anaphylaxis); Health hazards; Criminal charges (in deliberate cases); Lawsuits. |
| False Origin Claims | Labeling product from specific country/region when sourced elsewhere. | Fines; Regulatory action; Product recall. | Erosion of consumer trust; Damage to reputation of genuine local producers; Economic impact on honest businesses. |
| Ambiguous “Natural” Claims | Products labeled “natural” containing synthetic chemicals, artificial preservatives, or unexpected additives. | Lawsuits from consumers; Regulatory action. | Consumer deception; Erosion of trust; Financial damages. |
| Incorrect Dates | Wrong production or expiry dates; Dates prone to falling off. | Product recall; Regulatory penalties. | Safety risks (consumption of unsafe food); Loss of consumer confidence. |
Precision and Performance: How Advanced Machinery Ensures Labeling Compliance
Modern packaging and labeling machinery, including advanced features, are indispensable for achieving and maintaining compliance at scale. Investing in these technologies transforms compliance from a reactive burden into a proactive, embedded process, turning an operational cost into a strategic investment in business continuity, brand equity, and competitive advantage. Manufacturers are not merely acquiring machines; they are investing in a “compliance fortress” that protects their market access and long-term profitability.
Automated Labeling Machines: Boosting Accuracy and Efficiency
Manual labeling processes are inherently prone to inaccuracies and are time-consuming. Automated labeling machines, however, revolutionize packaging operations by significantly increasing speed, accuracy, and overall efficiency. These systems ensure consistent label placement, which minimizes waste and boosts output. By standardizing processes, automated systems reduce errors, thereby saving time and avoiding costly rework or regulatory fines. This shift allows employees to focus on higher-value responsibilities such as quality control and innovation. For high-volume production lines, fully automatic labelers deliver unparalleled speed and consistency, handling complex labeling tasks with minimal supervision. When selecting a labeler, key factors to consider include production volume, compatibility with various container types, and specific labeling requirements such as speed, precision, and compliance features.
 |
| SF-3030 Automatic round bottle labeling machine |
Vision Inspection Systems: Real-time Error Detection and Quality Assurance
Beyond the capabilities of the human eye, advanced vision systems utilize high-resolution cameras, precision sensors, and intelligent software algorithms to inspect, monitor, and analyze products during production. These systems automate quality control processes with unparalleled accuracy and speed, playing a pivotal role in ensuring product safety, compliance, and quality in the highly regulated food industry.
Vision systems are crucial for ensuring compliance by detecting misaligned labels, guaranteeing proper positioning and adherence to both aesthetic and regulatory standards. They identify smudge prints, illegible text, incorrect product data, and color inconsistencies, verifying that labels are legible and compliant with regulations. Barcode validation is another critical feature, ensuring products can be accurately tracked and scanned throughout supply chains. These systems also detect missing labels, misprints, and other physical defects. Advanced AI-backed solutions are capable of delivering consistent inspection results even at high production speeds, addressing concerns about keeping up with demanding production targets. Vision systems integrate seamlessly into production lines, allowing for real-time rectification of issues, minimizing downtime, and preventing non-compliant products from reaching the market.
Data Integration and Serialization: Enhancing Traceability and Regulatory Adherence
Smart labeling systems provide each product with a unique digital identity through RFID, barcodes, or QR codes, enabling real-time tracking from the packaging station to the retail shelf. This capability is fundamental for end-to-end traceability, offering comprehensive supply chain visibility from raw material sourcing and ingredient tracking through processing methods to finished products. Such traceability is crucial for enabling rapid recalls, as it allows for quick identification of contamination sources and targeted recalls, thereby reducing health risks and limiting financial losses. It also aids in anti-counterfeiting efforts and provides consumers with detailed product origin and ingredient information, fostering transparency.
Serialization, common in pharmaceutical packaging, involves encoding lot numbers and expiry dates directly into 2D barcodes or RFID tags for precise tracking and regulatory compliance. While primarily discussed in a pharmaceutical context, this highlights capabilities directly relevant to food safety and traceability. Smart labeling systems often integrate with Manufacturing Execution Systems (MES) or Enterprise Resource Planning (ERP) databases, ensuring seamless synchronization between production data and labeling output. This integration enhances inventory accuracy and reduces waste, with automated compliance checks cross-referencing labels against regional regulations. These systems also allow for flexible customization, dynamically printing variable information such as lot numbers, expiration dates, or region-specific details on the fly, which is vital for personalization, batch changes, and multilingual labeling.
The “Packaging Line” as a Compliance Fortress
The entire packaging line, encompassing filling, sealing, and labeling, plays a pivotal role in ensuring product safety, quality, and regulatory compliance. Modern packaging lines are designed as integrated solutions, incorporating precision equipment, high-tech sensors, and quality-control measures to detect anomalies and contaminants, thereby ensuring that products are safe and fresh when they reach consumers.
The emergence of digital labeling, often through QR codes for expanded information , represents a response to the limitations and challenges of physical labels, such as the need to convey detailed information that might not fit legibly on small packaging. This suggests a future where physical labels serve as a concise, legally compliant gateway, while digital platforms provide deeper, dynamic, and consumer-demanded transparency (e.g., detailed sourcing, sustainability data). For manufacturers, this implies that packaging lines must not only apply physical labels with precision but also integrate capabilities for digital coding and data management, creating a hybrid compliance strategy that leverages both physical and digital realms.
Furthermore, the adoption of modular labeling systems offers crucial flexibility and scalability, allowing businesses to adapt to new products, changing regulations, and increasing demand without frequent equipment overhauls. This adaptability is key to navigating the “global-local” paradox discussed earlier, enabling manufacturers to future-proof their operations and remain agile in a fast-paced market.
Strategic Imperatives: Best Practices for Sustained Compliance
Achieving and consistently maintaining food labeling compliance in a dynamic regulatory environment requires strategic planning and continuous adaptation.
Proactive Regulatory Monitoring and Adaptation
Regulations are in a constant state of evolution, necessitating continuous vigilance and regular updates to knowledge of industry standards. Manufacturers must go beyond merely reacting to current rules; they need to anticipate and adapt to emerging trends. For global operations, managing multi-language and regional labeling requirements adds significant complexity and risk, demanding a robust global-local strategy that accounts for specific jurisdictional nuances.
Investing in the Right Technology: Choosing Your Labeling Partner Wisely
Embracing automated labeling machines is essential for boosting efficiency, accuracy, and error reduction in labeling processes. It is crucial to prioritize labelers equipped with advanced features such as sensor technology, vision systems, and sophisticated control systems to ensure flawless label placement and consistent quality. The chosen equipment should seamlessly integrate with existing production lines and ERP systems to create a cohesive workflow and enable real-time data synchronization. Opting for modular systems offers scalability and flexibility, allowing businesses to adapt to changing product lines, packaging types, and production volumes without extensive retooling.
Comprehensive Training and Robust Quality Control Protocols
Compliance cannot be siloed within a “regulatory department”; it must be ingrained across the entire organization. This means that every employee understands their role in upholding standards, fostering a proactive environment where errors are prevented rather than merely detected, and ensuring that all aspects of product development and marketing align with regulatory and ethical principles. This transforms compliance from a departmental responsibility into a fundamental organizational culture.
Regular and frequent workshops on food safety standards, allergen handling, and sanitation are vital for employee training. Properly trained operators can efficiently use labeling machinery and troubleshoot common issues, reducing errors and contributing to smooth operations. Manufacturers should develop and rigorously maintain Hazard Analysis and Critical Control Points (HACCP) plans to identify and control risks at every stage of production. Implementing robust quality control checks, including visual inspections, measurements, and verifications, ensures that labels are accurately positioned, aligned, and free from defects. Furthermore, continuously monitoring and analyzing the performance of labeling machinery using data and analytics helps identify potential issues early, enabling continuous improvement initiatives.
Designing for Legibility and Clarity: Beyond the Regulations
Beyond meeting minimum regulatory requirements, labels should be designed with optimal legibility and clarity in mind. This involves ensuring fonts are readable, information is easy to locate, and there is sufficient color contrast between text and background. Using plain language, short sentences, bullet points, and subheadings significantly enhances readability and user engagement. Product names must accurately reflect ingredients, and any artificial elements or flavors must be clearly disclosed. Manufacturers should exercise caution with ambiguous terms like “natural” and ensure that all claims made on packaging are accurate, verifiable, and fully compliant with relevant guidelines.
Future-Proofing Your Operations: Emerging Trends in Food Labeling and Packaging
The future of food labeling will be shaped by evolving consumer demands and technological advancements. Staying ahead of compliance means proactively understanding and responding to consumer sentiment, as these trends often precede and influence regulatory changes. Manufacturers who align their labeling and packaging strategies with emerging consumer values are not only gaining market share but also positioning themselves to meet future regulatory requirements more seamlessly, turning compliance into a competitive differentiator.
The “Clean Label” Movement: Transparency and Natural Ingredients
Globally, consumers are increasingly prioritizing transparency, health, and sustainability, leading to a strong demand for “clean label” products that feature recognizable, minimal, and natural components. Key consumer demands include “no artificial ingredients,” “no additives,” “only natural ingredients,” and “organic sourcing”. Consumers are meticulously scrutinizing ingredient lists, with nearly three out of four reconsidering purchases based on this information, emphasizing the importance of clear information on ingredients and sourcing. This trend necessitates prominent packaging claims and overt nutritional information. Innovation in this area is shifting towards highlighting the specific number of ingredients on labels to create simpler, more understandable lists.
Accessible Packaging: Catering to an Aging and Diverse Consumer Base
Demographic shifts, particularly aging populations, are driving a growing need for packaging that accommodates various physical limitations, such as dexterity issues, vision impairments, and other age-related conditions. This trend influences packaging design, leading to increased demand for ergonomic container shapes, easy-grip closures, tactile markings, and larger font sizes. Technological solutions are also emerging; for example, technologies like NaviLens, as implemented on Kellogg’s cereal boxes, allow consumers to access product information from a distance, which is particularly beneficial for those with vision impairments.
Sustainability and Ethical Claims: Building Consumer Trust
Consumer concern for sustainability is on the rise, with a growing preference for products that demonstrate sustainable sourcing, local production, and eco-friendly packaging. Retail sales of packaged food products with sustainability claims are growing significantly, underscoring the need for manufacturers to clearly communicate their environmental efforts on packaging. Consumers are seeking greater transparency regarding ingredient sourcing, traceability, and overall sustainability practices. Beyond environmental factors, ethical considerations such as fair trade and animal welfare are also increasingly influencing purchasing decisions.
The Rise of Digital and Smart Labeling: QR Codes and Beyond
Digital labeling systems, such as QR codes, are becoming more prevalent, allowing consumers to scan packaging for expanded product information via mobile devices. This innovation directly addresses concerns regarding small font sizes on physical labels and enhances accessibility. Consumers are increasingly utilizing technology, including scanning apps, to obtain detailed information about product origin, healthiness, environmental impact, and provenance. Smart labels provide dynamic, real-time information about a product’s identity, status, location, or condition as it moves through production and distribution, offering unprecedented levels of transparency and traceability.
Conclusion: Partnering for Packaging Compliance Excellence
Compliant food labeling is not merely a regulatory hurdle but a strategic imperative that underpins consumer trust, public health, and market access. The complexities of global regulations, coupled with the severe risks of non-compliance, necessitate a proactive and technologically advanced approach. Advanced packaging and labeling machinery, equipped with automation, vision inspection systems, and data integration capabilities, play a transformative role in ensuring precision, efficiency, and unwavering compliance.
Achieving and maintaining excellence in food labeling compliance demands strategic planning, continuous adaptation to evolving regulations and consumer trends, and the right technological partners. By embracing these principles and investing in cutting-edge solutions, manufacturers can navigate the intricate global landscape, safeguard their brand reputation, and ultimately thrive in a competitive market.
Food Labeling Compliance Packaging Guide: FAQs
Here are 10 frequently asked questions based on the article’s content:
Q1.What are the primary differences in food labeling compliance globally?
A: Food labeling compliance varies significantly across countries like the US, EU, and China. Key differences include requirements for common name/identity, net quantity, ingredient lists, nutrition information, allergen labeling, font sizes, manufacturer details, date formats, and prohibited claims.
Q2.What information is required for the “Common Name/Identity” on food packaging?
A: In the US, it must be prominently displayed and at least half the size of the largest print. The EU requires a clear legal designation of the product’s precise nature, while China mandates that it reflects the true nature and is not misleading.
Q3: How should “Net Quantity” be displayed on food packaging in different regions?
A: The US requires it on the Principal Display Panel (PDP) with a “Net Weight” prefix, located in the bottom 30%. The EU specifies the number of items, weight (g/kg), or volume (ml/l). China requires specific units (volume/mass/length) based on food state, and drained matter for solid/liquid phase foods.
Q4: What does the “Clean Label” movement mean for food packaging, as mentioned in the article?
A: The “Clean Label” movement reflects growing consumer demand for transparency, health, and sustainability. It pushes manufacturers to highlight “no artificial ingredients,” “no additives,” “only natural ingredients,” and “organic sourcing” on labels, emphasizing simpler, more understandable ingredient lists.
Q5: How is accessible packaging addressing the needs of an aging and diverse consumer base?
A: Packaging design is evolving to include ergonomic shapes, easy-grip closures, tactile markings, and larger font sizes to accommodate physical limitations. Technologies like NaviLens, which allow consumers to access product information from a distance, also benefit those with vision impairments.
Q6: What role do sustainability and ethical claims play in building consumer trust through food labeling?
A: Consumers increasingly prefer products demonstrating sustainable sourcing, local production, and eco-friendly packaging. Clearly communicating environmental efforts, ingredient traceability, overall sustainable practices, and ethical considerations like fair trade and animal welfare on packaging is crucial for building trust.
Q7: How are digital and smart labeling systems (e.g., QR codes) changing the way consumers access food information?
A: Digital labeling systems, such as QR codes, are becoming widespread, allowing consumers to scan packaging for expanded product information via mobile devices. This addresses concerns about small font sizes on physical labels and enhances accessibility, offering dynamic, real-time data on a product’s identity, status, and origin.
Q8: What are the severe repercussions of non-compliance with food labeling regulations?
A: Failing to meet food labeling requirements can lead to severe consequences beyond simple fines, including costly product recalls, damage to brand reputation, and potential legal action.
Q9: What are the specific requirements for “Allergen Labeling” in the US, EU, and China?
A: In the US, allergens are part of the ingredient list with clear disclosure. The EU mandates emphasis (bold, font, color) on 14 major allergens in the ingredient list. China requires 8 major allergens to be highlighted (bold, underline, or statement) and cautions against “allergen-free” claims.
Q10: What are the specific requirements for production and expiration dates on food labels in China?
A: China requires both the production date and shelf-life expiration date to be clearly displayed with high contrast in a dedicated area. For products with a shelf life exceeding six months, the production date may be omitted.
| References: | |
| 1. | Guidance for Industry: Food Labeling Guide ——Retrieved from:U.S. Food and Drug Administration – FDA |
| 2. | General Standard for the Labeling of Prepackaged Foods ——Retrieved from:State Administration for Market Regulation – SAMR |
| 3. | General Standard for the Labelling of Prepackaged Foods (CXS 1-1985) ——Retrieved from:Food and Agriculture Organization of the United Nations – FAO |






Comments